NervGen Pharma Reports Positive Topline Data from the Chronic Cohort of its Phase 1b/2a Clinical Trial Evaluating NVG-291 in Spinal Cord Injury

This news release constitutes a “designated news release” for the purposes of NervGen’s prospectus supplement dated December 19, 2024 to its short form base shelf prospectus dated November 25, 2024.
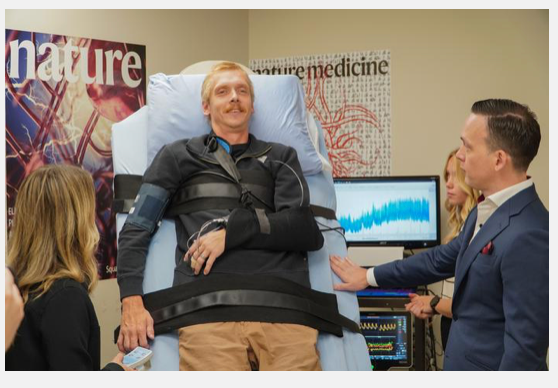
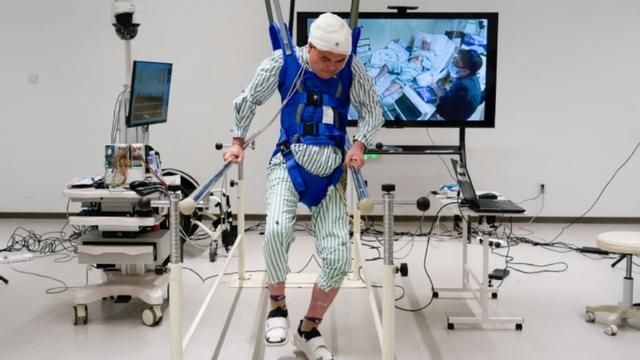
Vancouver, Canada, June 2, 2025 – NervGen Pharma Corp. (TSXV: NGEN) (OTCQB: NGENF), a clinical-stage biotech company dedicated to developing neuroreparative therapeutics, today announced positive topline results from the chronic cohort (1-10 years post injury) of its Phase 1b/2a clinical trial evaluating its lead drug candidate, NVG-291, as a potential treatment for spinal cord injury. NVG-291 met one of its co-primary endpoints and demonstrated promising changes in “GRASSP” score, a measure designed specifically to assess hand function in individuals with cervical SCI.
Topline results from the trial support the potential of NVG-291 to promote nervous system repair. The trial met a co-primary endpoint demonstrating improved motor connectivity in individuals with cervical chronic SCI receiving NVG-291 (n=10) compared to placebo (n=10). Data showed that subjects receiving NVG-291 achieved a three-fold increase in the strength of motor connectivity to an important hand muscle (first dorsal interosseus), as measured by change in the normalized motor evoked potentials (MEP) amplitude. (Baseline/Week 12 actual results: 6.207/18.773 for NVG-291 vs. 6.527/7.760 for placebo, p-value 0.0155). The second co-primary endpoint evaluating connectivity in a leg muscle (tibialis anterior) did not achieve statistical significance. The co-primary endpoint approach to the trial design is intended to permit only one of the co-primary endpoints to achieve statistical significance, though with a more rigorous p-value of <0.025 being required.
Read full article
here